INFORMACIÓN EXCLUSIVA PARA: MEDICOS, PARAMEDICOS, ESTUDIANTES DE MEDICINA.PRINCIPALMENTE. LOS INTERESADOS EN LA INFORMACION AQUI PLASMADA(LES RECORDAMOS QUE ESTA ES UNA INFORMACION MUY ESPECIALIZADA)EXCLUSIVAMENTE PARA EL ANALISIS POR MEDICOS Y AFINES,CON FINES EDUCACIONALES,TOMADOS DE LA LITERATURA INTERNACIONAL. ARTICULOS EN INGLES Y EN ESPAÑOL.
martes, 3 de noviembre de 2009
La Cirugia minimamente Invasiva ,causa mas incontinencia y mas disfuncion erectil que la Prostatectomia abierta retropubica?
Cual sera la realidad?
Pronto tenedremos los numeros necesarios para aclarar esta controversia.
Lo que si es cierto en nuestras manos ,que solo hacemos Prostatectomia Abierta retropubica y en la cual tenemos una experiencia en el pais abundante,podemos decir que nuestro tiempo de operacion es corto(una hora hora y media)incontinencia,en nuestros primeros pacientes muy leve y los costos son mucho menores que las otra cirugias y la radioterapia,con resultados a 12 años muy buenos.
Disfuncion erectil: esperando a los 5 meses,tenemos que el 75% se recuperan y nos queda un 25% que no se recupera,pasarian a beneficiarse de uan protesis peneana.
DR.Ricardo Szemat Nikolajenko.Urologo.04142570173
Varicocele combinado con fumar empeora la motilidad y la morfologia
NEW YORK (Reuters Health) Oct 26 - Smoking combines with varicocele to worsen sperm motility and morphology, say researchers from Italy in the October Urology.
"The present study indicates a negative synergic effect of cigarette smoking and varicocele: another strong reason to stop smoking," Dr. Elena Moretti from the University of Siena told Reuters Health. "The main message of the present research should be to implement effective interventions targeted to encourage men to quit smoking in order to improve general health and also their fertility potential."
Dr. Moretti and colleagues investigated whether smoking increases the adverse effects of varicocele on sperm morphology and function in a study of 121 smokers and 158 nonsmokers with varicocele.
Progressive sperm motility was lower in both groups compared to World Health Organization values, the authors report, but the difference between smokers and nonsmokers was not significant.
Similarly, the percentage of immaturity, necrosis, and apoptosis were significantly lower than reference values in men with varicocele, but the values did not differ significantly between smokers and nonsmokers.
When the smokers were categorized according to their daily cigarette usage, however, sperm concentration, progressive motility, apoptosis, immaturity, and necrosis were all significantly worse among heavy and moderate smokers than among mild smokers.
"I believe that the most important point to be stressed is to persuade males to quit smoking as soon as possible, as it has been reported that reduced fecundity associated with smoking may in great part be reversed within a year of smoking cessation," Dr. Moretti said.
"The study of the relationship between smoking and varicocele, which we have shown to affect sperm characteristics, is the first step in the research we have undertaken to discover the determinants of sperm injury," Dr. Moretti added. "It should be of pivotal interest to consider other putative mechanisms of damage, in addition to free oxygen species release due to smoking and the presence of varicocele (widely reported in the literature)
Urology 2009;74:794-800.
Comentario RSZN; El dr moretti piensa que esta es la primera etapa en esa investigacion para descubrir las determinantes en la injuria del espermatozoide y buscar otros mecanismos de daño,aparte de la liberacion de " especies oxigeno libre" debido al fumar y la presencia de Varicocele.
DR Ricardo Szemat Nikolajenko;urologo.
sábado, 12 de septiembre de 2009
nueva clasificacion de los tumores renales
RIO DE JANEIRO, BRAZIL (UroToday.com) - Clear cell carcinoma occurs in 60% of resected adult tumors. They are yellow tumors with nests of clear cytoplasm and express many growth factors including VEGF, EGFR and Carbonic anhydrase. VHL gene mutations are present.
Papillary cancers comprise 15% of resected adult tumors. Type I has very good survival and type II poor survival. It is unclear if they are really distinct entities molecularly.
Chromophobe renal tumors comprise 5% of adult renal tumors and they have a greater than 90% 10-year survival. While they can have very aggressive sarcomatoid variants, it is rare. They are generally unifocal tumors with large cells that can be confused with oncocytomas due to large amount of pink cytoplasm.
Collecting duct tumors are seen in less than 1% of all renal tumors. They occur in young patients and are very aggressive. They are infiltrative versus circumscribed and virtually indistinguishable from urothelial cancer or renal pelvic tumors. Medullary tumors occur only in patients with sickle cell trait and are uniformly lethal within 6 months.
Finally, mucinous tubular and spindle cell tumors are new entities in the classification system. They are low grade and occur predominately in women.
Presented by David Grignon, MD at the VI Maratona Urológica do Rio de Janeiro - August 14 - 15, 2009.
miércoles, 2 de septiembre de 2009
evaluacion del papel del sistema sertoninergico en el control del musculo liso de la vesicula seminal humana.Una manera de entenderla in vitro.
ABSTRACT
Introduction. It has been suggested that serotonin re-uptake inhibitors (SRIs) may retard the ejaculatory response by acting directly on the seminal vesicle (SV) and ductus deferens smooth muscle. However, until now, only a very few experimental studies have investigated such potential local (peripheral) effects.
Aim. To elucidate the effects of serotonin (5-HT) and the SRIs clomipramine, fluoxetine and imipramine on the tension induced by norepinephrine (NE) of isolated human SV smooth muscle, as well as on the production of tissue cyclic AMP and cyclic GMP.
Main Outcome Measures. To measure the inhibition exerted by serotonin and SRIs clomipramine, fluoxetine, and imipramine on the contractile response of isolated SV tissue. In addition, the effects of the drugs on the turn-over of cyclic nucleotides cAMP and cGMP were also elucidated.
Methods. The effects of the cumulative addition of serotonin and the SRIs clomipramine, fluoxetine and imipramine (1 nM–10 µM) on the tension induced by the alpha
Results. The tension induced by NE was dose-dependently reversed by the drugs tested. The rank order of efficacy was: imipramine ≥ fluoxetine ≥ clomipramine > serotonin. Mean reversion of tension was measured between 66 ± 6.6% and 52 ± 6.6%. These effects were paralleled by a 1.3-fold to 2.7-fold increase in tissue cAMP in response to exposure to the drugs. In contrast, no significant enhancement in cGMP was noted.
Conclusions. The findings, for the first time, present evidence that SRIs may antagonize the sympathetic contraction of SV smooth muscle via stimulation of tissue cyclic AMP. Birowo P, Ückert S, Kedia GT, Scheller F, Meyer M, Taher A, Rahardjo D, Jonas U, and Kuczyk MA. Evaluating the role of the serotoninergic system in the control of human seminal vesicle smooth muscle—An in vitro approach. J Sex Med **;**:**–**.
domingo, 30 de agosto de 2009
La vacuna del VPH(virus de papiloma Humano)puede prevenir muchos casos de carcinoma de Pene
"Disponible vacunas contra el VPH es probable que sea eficaz en los tumores de pene," la Dra. Silvia de Sanjosé y colaboradores sugieren en el Journal of Clinical Pathology, publicado en línea el 25 de agosto.
El carcinoma de pene es relativamente rara en los países desarrollados, que representan menos del 1% de los cánceres de adultos varones en Europa y América del Norte, señalan los autores. La incidencia es mucho mayor en otras regiones, causando hasta el 10% de todos los tumores malignos en los hombres en América del Sur, África y Asia.
Para examinar el papel del VPH en determinados subtipos histológicos de cáncer de pene, el Dr. de Sanjosé, del Instituto Catalán de Oncología de Barcelona, España, y su equipo realizó una revisión bibliográfica sistemática y exhaustiva de los principales estudios de cáncer de pene, publicados entre 1986 y 2008.
Incluido en su análisis fue de 31 estudios que incluyeron 1466 pacientes con carcinoma de pene. La prevalencia global de VPH fue 46,9%, la mayoría de los cuales eran los tipos de alto riesgo cubierto por la vacuna contra el VPH actual: HPV-16 (60%), HPV-18 (13%), y HPV-6/11 (8%) .
"Basaloide y verrugosas carcinomas de células escamosas fueron el VPH más frecuentes relacionadas con los tipos histológicos", señala el estudio en equipo ", pero queratinizado y no queratinizado subtipos también mostraron tasas de prevalencia de alrededor del 50%."
El Dr. de Sanjosé y colaboradores señalan que la vacuna profiláctica contra el VPH en los hombres parece ser segura e inmunogénica. Ellos proponen que, "a pesar de carcinoma de pene es una enfermedad rara, alrededor de 7000 casos podría evitarse anualmente por la erradicación de VPH 16/18."
J Clin Pathol 2009.
El uso de la hemoglobina A1C para el diagnostico de Diabetes.
une 7, 2009 (New Orleans, Louisiana) — The American Diabetes Association (ADA), the International Diabetes Federation (IDF), and the European Association for the Study of Diabetes (EASD) have joined forces to recommend the use of the hemoglobin A1C assay for the diagnosis of diabetes.
The international expert committee's recommendations were announced here on Friday during the opening hours of the ADA's 69th Scientific Sessions and released simultaneously online in the July issue of Diabetes Care.
"This is the first major departure in 30 years in diabetes diagnosis," committee chairman David M. Nathan, MD, director of the Diabetes Center at Massachusetts General Hospital and professor of medicine at Harvard Medical School in Boston, declared in presenting the committee's findings.
"A1C values vary less than FPG [fasting plasma glucose] values and the assay for A1C has technical advantages compared with the glucose assay," Dr. Nathan said. A1C gives a picture of the average blood glucose level over the preceding 2 to 3 months, he added.
"A1C has numerous advantages over plasma glucose measurement," Dr. Nathan continued. "It's a more stable chemical moiety.... It's more convenient. The patient doesn't need to fast, and measuring A1C is more convenient and easier for patients who will no longer be required to perform a fasting or oral glucose tolerance test.... And it is correlated tightly with the risk of developing retinopathy."
A disadvantage is the cost. "It is more expensive," Dr. Nathan acknowledged. However, cost analyses have not been done, "...and costs are not the same as charges [to the patient]."
The committee has determined that an A1C value of 6.5% or greater should be used for the diagnosis of diabetes.
This cut-point, Dr. Nathan said, "is where risk of retinopathy really starts to go up."
He cautioned that there is no hard line between diabetes and normoglycemia, however, "...an A1C level of 6.5% is sufficiently sensitive and specific to identify people who have diabetes."
"We support the conclusion of the committee, that this is an appropriate way to diagnose diabetes," stated Paul Robertson, MD, president of medicine and science at the ADA and professor of medicine at the University of Washington in Seattle.
"Now, we have to refer the committee's findings to practice groups for review of the implications and for recommendations," Dr. Robertson told Medscape Diabetes & Endocrinology after the committee's presentation.
"We purposely avoided using estimated average glucose, or EAG, as this is just a way to convert the A1C into glucose levels.... And one thing we want to try to get away from is the term prediabetes," Dr. Nathan said. "It suggests that people with it will go on to get diabetes, but that is not the case."
"We don't know if we will be diagnosing more patients with diabetes or less, with AIC," Dr. Nathan commented. Cut-off values or practice guidelines have not been established. More study needs to be done first, but "physicians should not mix and match A1C and blood glucose levels. They should stick with one in reviewing a patient's history," Dr. Nathan asserted.
"There is no gold standard assay," said session moderator Richard Kahn, PhD, chief medical and scientific officer of the ADA, which is headquartered in Alexandria, Virginia. "All of these tests measure different things. They all have value. But A1C is the best test to assess risk of retinopathy."
"We [the ADA] are not issuing a position statement at this time," Dr. Robertson stressed when speaking withMedscape Diabetes & Endocrinology. "It is too soon to write a position paper yet. We need to know what we are getting into first."
"Some parts of the world are not going to be able to use this," Dr. Robertson added. "It may be too expensive to use in the developing world. Some of these countries have severe chronic anemia, hemolytic anemia, and so on, where we will have to fall back on traditional tests. We are being very cognizant of the international implications." A1C assays are inaccurate in cases of severely low hemoglobin levels.
"We don't think physicians will have a hard time adopting the test...a lot of them are doing it already. We think it will only take a couple of years to be adopted widely into clinical practice," Dr. Kahn told Medscape Diabetes & Endocrinology. "Physicians won't be shocked by this report, but patients — and insurance companies — might be. There are wider social issues that haven't been looked at yet."
None of the speakers at this session disclosed any relevant financial relationships.
American Diabetes Association (ADA) 69th Scientific Sessions. Presented June 5, 2009.
Diabetes Care. Published online June 5, 2009.
TEl uso de |
viernes, 28 de agosto de 2009
la circuncision quita las celulas de Langerhans ,buscadas por el HIV
om Reuters Health Information
Circumcision Removes Langerhans Cells Targeted by HIV
|
By Martha Kerr
CHICAGO (Reuters Health) May 01 - Circumcision protects against HIV infection because the foreskin contains a high density of Langerhans cells, an established avenue of HIV infection, urologists reported here at the American Urological Association annual meeting.
The finding comes from a study conducted at the Royal Victoria Hospital and the University of Melbourne, Australia. "Our aim was to determine the...Langerhans cell distribution of the remnant foreskin epithelium in circumcised adult men, and compare this to the epithelium of the inner foreskin and penile shaft," Dr. Sandra L. Hallamore noted.
She pointed out that circumcision creates a "remnant foreskin -- a small cuff of skin around the base of the glans penis." The investigators took 2 mm biopsies from the inner foreskin of 10 uncircumcised men, and from the remnant foreskin and penile shaft of 10 circumcised men.
The team found that inner foreskin has a significantly higher density of Langerhans cells than residual areas of the foreskin.
"The removal of this high density of HIV target cells, and the subsequent formation of a remnant foreskin with low Langerhans cell density, may explain the reduction in HIV transmission with adult circumcision," Dr. Hallamore told attendees. "The reduction in HIV transmission does not appear to be related to a thinner or poorly keratinized inner foreskin epithelium," she added.
la experiencia de las parejas sexuales de las personas con ´Cáncer; resultados
The Experience of Sexual Partners of Persons With Cancer: Results
One hundred twenty-two participants (43 men, 79 women), or 78% of this subsample, reported that the onset of cancer had negatively impacted upon their sexuality and their sexual relationship. When we examined the type of cancers associated with changes to sexuality after cancer, the rate was 90% for partners of men with prostate cancer, 71% for partners of women with gynecologic cancer, and 78% for partners of women with breast cancer. Overall, the percentage of partner carers of partners with "nonreproductive" cancers who reported an impact on the sexual relationship was 76%, and the percentage of those caring for partners with cancers involving reproductive sites was 84%.
Each of the 122 participants elaborated on the changes to his or her sexual relationship experienced after cancer in open-ended responses. These responses concerned the status of the sexual relationship, perceived reasons for the changes, and partners' feelings about the changed relationship. Each theme is reported below, illustrated by extracts from the open-ended questionnaire items and the interviews. Demographic information is provided for longer quotes stemming from the interviews. For readability, these specific details are not provided for every open-ended questionnaire quote. Percentages cited refer to the open-ended questionnaire responses.
Status of Current Sexual Relationship
Two major themes characterized accounts of the current status of the sexual relationship: cessation or decreased frequency of sex or intimacy and renegotiation of sex or intimacy.
Cessation or Decreased Frequency of Sex and Intimacy. A complete cessation of sex or a marked decrease in the frequency of sex was reported by 59% of the women and 79% of the men. For those who experienced a complete cessation of sex, the "end" of the sexual relationship was reported as a sudden event: "[o]ur sex life disappeared overnight" and "[g]one from fantastic sex life to none." For other participants, it was a gradual change: "[i]nitially we found other ways to be intimate, however, over time our sex life has ceased." The impact of both the cessation of sex and the loss of intimacy was evident in the following interview extract:
A big... big chunk of your life is lost, and I don't just mean the physical aspects of it... I mean that's... you can live with that or you can... or go without, but... the whole package is gone and I think that's hard that, you're a widow with somebody that's still around. [57-year-old woman caring for 53-year-old husband with brain cancer]
Of the participants who reported decreased sexual frequency rather than a complete cessation, many positioned their sexual relationship in ways that indicated that they had previously enjoyed an active sex life: "[w]e had a very strong physical relationship up until the cancer was discovered and after it, it just faded away" and "[v]ery poor, we use to have sex 5 times a week, now maybe once in 3 or 4 months." Others simply described a change in frequency: "[v]irtually non-existent" and "[t]his aspect of our marriage has nearly stopped." Many of the participants who reported cessation or decreased frequency of sex also reported decreased closeness and intimacy. Responses included the following: "I couldn't cuddle like we used to" and "[o]ften feel frustrated that it doesn't happen like it used to-he is not as romantic either."
Renegotiation of Sexual and Nonsexual Intimacy After Cancer. A renegotiation of their sexual relationship to include noncoital sexual practices or the development of nonsexual intimacy was reported by 19% of the women and 14% of the men. Men (12%) were more likely than women (1%) to report having developed alternative sexual behaviors to those practiced before the patient had cancer. These behaviors consisted of changed sexual positions when attempting intercourse: "I am obviously more careful, having adjusted positions," and the development of "workable alternatives to achieve partner satisfaction... within restrictions caused by the treatments," including oral sex, massage, masturbation, or the use of a vibrator.
Women (18%) were more likely than men (5%) to report that renegotiation involved nonsexual intimacy such as hugging and cuddling: "I'd put my legs up on his lap, and he'd put his arms around me, and I'd cuddle into him, and we'd watch TV."
The last week of my husband's life, he wanted to make love, but physically could not due to his illness. We talked this over as we always did and he knew that hugs, cuddles, and closeness were far more important than the actual act of making love. [64-year-old woman who cared for 64-year-old husband with pancreatic cancer, bereaved]
The importance of closeness to the well-being both of the partner and the person with cancer was emphasized by many of the interviewees. In the excerpt below, one partner describes how important it was to maintain physical closeness with her husband, despite the significant physical barriers that could have served to restrict the expression of intimacy.
We deliberately had kept the double bed. And then, when he got sick, and they needed a more supportive bed, I brought my single bed in, and we got this special height, set at the same height, so that he was always next to me.... I remember the morning he died, I remember cuddling him all night. (...) Just to have your... to have your arm around him was just so, so good. [59-year-old woman who cared for 69-year-old husband with mesothelioma, bereaved]
Reasons for Changes in Sexual Relationships
Many of the participants provided reasons for changes in their sexual relationship after cancer including most notably the impact of cancer treatment, exhaustion resulting from the caring role, and repositioning of the person with cancer as a patient rather than as a sexual partner.
Impact of Cancer Treatment. Cancer treatments were positioned as the primary reason for changes to the sexual relationship. The effects of the treatments meant that there were now physical barriers to sex, which were reported by 30% of the men and 33% of the women. For example, "[h]ormonal treatment has the effect of chemical castration, ie, my husband has no sexual function"; "her poor body has been so cut and chemo has affected her so much that sex is not even possible"; and "non-existent due mainly to the chronic pain syndrome and a less than full confidence in colostomy bags!" For others, cessation or reduction in sex was due to overall bodily restrictions: "[h]e is physically unable to position himself for sex now."
In June an epidural catheter was inserted into my husband's chest and commenced on morphine 30 mgs three times a day. Not only was there no energy or inclination, because of the pain and reduced energy, there was now a "physical barrier" to our relationship as well as all the side effects of morphine. [59-year-old woman who cared for 56-year-old husband with mesothelioma]
Many of the participants also described adverse effects of the treatment such as pain, fatigue, and exhaustion. As one woman participant said about pain, "[w]hen he is unwell because of treatment I tend to be very careful in touching him in case it causes further pain/discomfort." Descriptions of fatigue being given as a reason for changes to the sexual relationship included the following: "[a]s a result of treatment (chemotherapy) my wife is tired more of the time and her libido is reduced" and "[h]e was just too exhausted." The impact of cancer treatment on the self-esteem and self-image of their partner was also identified as a reason for changes to the sexual relationship in a number of cases. For example, one partner commented:
As her health declined she had very low self-esteem caused by loss of hair and muscle tone. When I did have sex at the beginning she would accuse me of not treating her the same as I did in the past and get depressed. [61-year-old man caring for 43-year-old female partner with lung cancer, bereaved]
Exhaustion Resulting from the Caring Role. Exhaustion resulting from the caring role was positioned as the cause of changes to their sexual relationship by 16% of the women and 9% of the men. The responses included the following: "[w]e don't really have any intimacy anymore for reasons including his health and my exhaustion"; "[e]xhaustion, brain still ticking about things to be organized"; and "[e]ven if he was still interested in the sexual side of our marriage I think I would have been too exhausted to have taken part." Participants also commented on a revised prioritization that centered on coping and survival, leaving no time for sex or intimacy.
The sexual issue is really not a priority as all our energy seems to be focused on trying to find a way to beat the cancer. [44-year-old woman caring for 58-year-old husband with prostate cancer]
The Repositioning of the Person with Cancer as a Patient. For 28% of the women and 47% of the men caring for a partner with cancer, the caring role was reported to have resulted in a repositioning of the person with cancer as a patient, which subsequently influenced their sexual relationship. Many partners described emotional effects of the caring role or concern for their partner's feelings and health status. Comments included the following: "[w]ith all the worry and stress that my husband is most likely to die, I now have very little desire for sex"; "[c]urbed by concerns about inflicting pain or discomfort"; and "I just wanted to treat her the same as I always did but I couldn't get the thought out of my head that she was terminally ill."
Participants also reported that they had redefined their role as a carer rather than as a "lover." Some examples are as follows: "[m]y role as a carer has overridden my role as a wife..." and "[h]aving to spend more time on house/garden chores and be carer/nurse, one feels more like a housekeeper than a lover."
When you are a carer it's hard to be a lover, for either party, when dealing with incontinence of both bowel and bladder infections, along with the daily grind of showering, dressing, shaving, etc, then transferring from bed to wheelchair and return. [59-year-old woman who cared for 63-year-old male partner with hematologic cancer]
A number of male participants gave accounts that suggested sex was inappropriate with a person with cancer: "I was very aware of my role as carer and never did anything to embarrass my wife. There was never any inappropriate behaviour." This could result in ambivalent feelings in the face of the partners' own desires, as the following account illustrates:
I feel disgusted with myself that I would inflict sex upon a dying woman, having said that my wife does not object and occasionally welcomes it, saying it is a life giving and loving act and a part of our sacrament.... I was never a fast lover, but now I try and get it over and done with for her. [45-year-old man caring for 44-year-old wife with breast cancer]
A number of the women participants also described positioning their partner as a child, a position that was seen as antithetical to sexuality: "it's like looking after... one of your children now."
Partners' Feelings About Their Changed Sexual Relationship
A number of the participants gave accounts of the emotions that they experienced in response to the changes in their sexual relationship after cancer, with accounts evenly divided between positive and negative feelings.
Positive Feelings. Accounts of positive feelings were provided by 17% of the women and 16% of the men. Many participants described feelings of understanding or acceptance of the effects of cancer or caring on their sexual relationship. Accounts included the following:
Treatment makes my partner feel sick and makes me worry about him so this means we don't feel up to sex... This is not an issue-just a fact/reality of current situation. [39-year-old woman caring for 53-year-old male partner with lung cancer]
He is not up to performing and he has talked to me about it several times, but I assure him that I understand. [66-year-old woman who cared for 66-year-old husband with colon cancer]
A number of participants also reported feelings of affection and companionate devotion:
Sexual urge had gone but my husband made me feel the most loved and cared for woman on this earth by his loving actions, his consideration, his caring attitude and the advice I sought even up till 12 hours before he died. I loved this man totally and he me. [68-year-old woman who cared for 69-year-old husband with brain cancer, bereaved]
[Husband] has multiple brain tumours, lung tumours and clots plus multiple liver tumours so I just hug and reassure that I am here for the "long haul" come what may. [66-year-old woman who cared for 66-year-old husband with colon cancer]
The cancer experience was positioned as having brought the couple closer together by some participants, with one man saying that he "probably has a more affectionate relationship at this point in our lives, and marriage" than before the onset of cancer and another commenting that "with the exclusion of sex, our intimacy is closer probably than it's been for a long time." Increased emotional closeness, despite absence of sex, was also evident in a number of the women participants' interviews:
We are so much closer now than we were... we wouldn't be as close now and we wouldn't be able to talk about absolutely anything now... Just seeing him at night, just makes my heart just go hshshsh... Whereas before I don't think we appreciated that about each other. [29-year-old woman caring for 33-year-old husband with brain cancer]
Negative Feelings. Accounts of negative feelings in response to changes to sexuality were reported by 13% of the women and 21% of the men. These feelings included sadness that their sexual relationship was "lost": "[t]here is just an enormous sadness that we can no longer have this intimacy..." and "[s]till this whole traumatic experience has left me feeling very upset." A number of participants also reported with self-blame, "[n]o sex for 12 months-more my fault," or rejection by their partners, "I felt excluded and unwanted. Sex became a chore and mechanical" and "[s]he has absolutely no sexual interest in me whatsoever."
[S]ometimes you feel guilty that you've got, you know, disgust about it or you know the thing now starts to rot and you feel disgusted by that. [61-year-old woman who cared for 52-year-old female partner with lung cancer]
I don't feel the desire to have a physical relationship with my husband. It almost makes me feel ill to even contemplate it. His whole physical appearance repels me. [52-year-old woman caring for 55-year-old husband with prostate cancer]
A lack of fulfillment in relation to sex was another common feeling: "[n]ot able to relax and enjoy"; "[o]ften feel frustrated that it doesn't happen like it used to"; "[a]t times, I have considered having an affair purely for sexual gratification"; and "leaves me less satisfied." Some participants mentioned feelings of perceived obligation. For men, it was usually in relation to feeling that their partners felt obliged to provide sex. Examples included the following: "[o]n the infrequent occasions we now have sex she wants it over and done with as quickly as possible" and "[s]he became less interested in sex and only accommodated me as if it was a wifey duty." For women participants, obligation was positioned in terms of themselves feeling obliged to engage in sex.
At the early stages of the diagnosis I felt that I couldn't say no to him which put a lot of pressure on me. I had to make sure that I could respond to him and not give him any chance of feeling that I didn't want to make love to him. [59-year-old woman caring for 63-year-old husband with gastric adenocarcinoma]
A small number of women participants shared negative feelings regarding family planning and fertility:
Prostate cancer has required removal of the sac that produces sperm. I am 36 and had always taken for granted I would fall pregnant in the most natural and intimate way. Once my partner is stronger, we will seek advice from an IVF Clinic regarding artificial insemination (hence my partner has secured enough in the sperm bank!). Still this whole traumatic experience has left me feeling very upset. [36-year-old woman caring for 59-year-old husband with prostate cancer]
Discussions of Sexuality With Healthcare Professionals
In response to a question regarding whether a healthcare professional had discussed sexuality with them, 20% of participants indicated that they had. The rate of discussion differed across cancer types, ranging from 50% of prostate cancer partner carers to 0% of respiratory cancer. The rates across the other main cancer types were 33% for brain, 33% for pancreatic, 30% for breast, 29% for gynecologic, 20% for multiple sexual, 17% for colorectal/digestive, 17% for mesothelioma, 15% for multiple nonsexual, 15% for other, and for 9% hematologic. Of those who had discussed sexuality with healthcare providers, only 37% indicated that they were satisfied or very satisfied.
In the interviews, a number of the partners commented on their discussions with healthcare professionals, in each case giving a critical account. When they asked about sexual matters, participants reported being told as follows: "[o]h you don't need to know that and things like that." They were told that they were "irresponsible to be thinking about having children" in raising fertility as a concern. Most, however, gave accounts of sexuality not being discussed at all: "I haven't got a lot of medical advice about how we should continue to conduct our intimate relationship" and "they did not educate us on anything... at all."
[I]t's not properly addressed by the medical profession, it is just completely glossed over. And I can remember, you know, we were sitting when the diagnosis came through and the guy said well, you know, you'll get these hormone pills and we'll give you an injection into your stomach and of course that will be the end of your sex life; and we're just sitting there (...) That was the end of the discussion. [67-year-old woman who cared for 85-year-old husband with prostate, bowel, and lung cancer, bereaved]
Intimidada y sexualidad luego del cáncer
Introduction
It is now widely recognized that cancer and its treatment can have a significant effect on the quality of life of both people with cancer and family members providing informal care, particularly their intimate partner.[1] Sexuality and intimacy are important aspects of quality of life,[2] and there is a growing body of evidence to show that cancer can result in dramatic changes to sexuality, sexual functioning, relationships, and sense of self.[3-5] These changes can be experienced as the most significant in the life of a person with cancer[6] and can lead to emotional distance between couples,[7] as well as feelings of isolation, anxiety, depression,[8] or inadequacy.[9]
Whereas the experiences of partners are often neglected in research on sexuality and intimacy after cancer,[10]there is growing acknowledgment of their unmet needs in this area.[11,12] Reported disruptions include decreases in their own sex drive, fear of initiating sex with their partner, difficulty regaining a level of "normality" within the sexual relationship, and feeling unwanted and unattractive because of cessation of sex.[4,13,14] It has also been argued that when sexual intercourse ceases in the context of illness, touching and other forms of affectionate physical contact also diminish[15] because of a perception among some couples that these forms of affection necessarily lead to sexual intercourse, which is either not possible or deemed inappropriate.[16]
One of the limitations of research in this area is the focus on experiences of sexuality after cancer that affects the reproductive organs.[4,13,14] There is a need for research examining the experiences of partners across a range of cancer types, as cancers that do not involve parts of the body designated as "sexual" or "reproductive" may also impact on sexuality.[10] A further related limitation is the focus on the physiological effects of cancer and its treatment upon the sexuality of partners. However, the dynamics of the caregiving relationship and social constructions surrounding what constitutes appropriate sexual conduct after cancer may also interfere with a couples' sexual relationship. For example, partners who provide a great deal of intimate physical care to the person with cancer (such as helping with toileting or feeding) can experience difficulties in continuing to see them as a sexual person[17] and reposition them as a "patient"[18] or as asexual.[19] Broader cultural constructions of normative sexuality may also be influential in determining the ability of couples to renegotiate sexuality and intimacy after cancer, particularly when sexual intercourse is no longer possible. As Judith Butler[20] has argued, our understanding of sexual subjectivity is confined within a "heterosexual matrix," within which masculinity and femininity are performed through engagement in normative sexual practices described as the "coital imperative,"[21]with failure to perform coitus positioned as "dysfunction" and other practices referred to as not "real sex."[22] This provides a theoretical framework for understanding why many heterosexual couples who cannot physiologically engage in sexual intercourse after diagnosis and treatment of cancer cease all expression of sexual intimacy. It also suggests that the dynamics and pressures of the caring role, as well as constructions and beliefs about what is acceptable or appropriate sexually after cancer, are worthy of investigation.
The aim of the present study was to examine the subjective experience of sexuality and intimacy after the diagnosis and treatment of cancer for partners of a person with cancer across a range of cancer types using qualitative methods within a critical realist epistemological standpoint. Advocated as the way forward for research examining health in a sociocultural context,[23] critical realism recognizes the materiality of the body and other aspects of experience (such as cancer, cancer treatments, the caring role) but conceptualizes this materiality as always mediated by culture, language, and subjectivity.[24] A variety of methodological approaches, both qualitative and quantitative, are valued equally within a critical realist approach, and there is acceptance of the legitimacy of subjective experience, often marginalized in mainstream health psychology research.
cambios en la sexualidad y en la intimidad luego del cáncer
Abstract
Changes in sexuality and intimacy after cancer were examined using open-ended questionnaire responses with 156 informal carers who were partners of a person with cancer. Interviews were conducted with 20 participants to examine changes in depth. Seventy-six percent of partners of a person with "nonreproductive" cancer types and 84% of partners caring for a person with cancer involving "reproductive" sites reported an impact on their sexual relationship. Cessation or decreased frequency of sex and intimacy was reported by 59% of the women and 79% of the men. Renegotiation of sexuality and intimacy after cancer was reported by only 19% of the women and 14% of the men. Reasons for changes to sexuality after cancer were the impact of cancer treatments, exhaustion due to caring, and repositioning of the person with cancer as a patient, not a sexual partner. Changes to sexuality were associated with reports of self-blame, rejection, sadness, anger, and lack of sexual fulfillment. Positive consequences of changes included accepting the changed sexual relationship and having increased closeness and intimacy. These findings reinforce the need to acknowledge the sexual needs of partners as well as people with cancer, by healthcare professionals working in cancer and palliative care.
jueves, 27 de agosto de 2009
Cáncer de Próstata-biologia,diagnostico,Patologia,estadiamiento e historia natural
eMedicine Specialties
Prostate Cancer - Biology, Diagnosis, Pathology, Staging, and Natural History
Dan Theodorescu, MD, PhD, Paul Mellon Professor of Urologic Oncology, Department of Urology, University of Virginia Health Sciences Center
Tracey L Krupski, MD, MPH, Assistant Professor, Department of Urology, University of Virginia
Updated: May 21, 2009
Introduction
Prostate cancer is the most common noncutaneous cancer among males. Lung and bronchial cancer account for 37% of cancer-related death in males; prostate and colon cancers account for another 10% each. The diagnosis and treatment of prostate cancer continue to evolve. With the development of prostate-specific antigen (PSA) screening, prostate cancer is being diagnosed earlier in the disease course. Although prostate cancer can be a slow-growing cancer, thousands of men die of the disease each year. Education is important to help men understand the risk of progression and the various treatment options. This article provides a current overview of the biology, pathology, diagnostic techniques, natural history, and screening of this disorder.
For excellent patient education resources, visit eMedicine's Prostate Health Center and Cancer and Tumors Center. Also, see eMedicine's patient education article Prostate Cancer.
Incidental Findings
In the modern era, most patients present because of abnormalities in a screening PSA level or findings on digital rectal examination (DRE) rather than because of symptoms (see Prostate-Specific Antigen). However, prostate cancer can be an incidental pathologic finding when tissue is removed during transurethral resection to manage obstructive prostatic symptoms (see Prostate Hyperplasia, Benign).
Elevated prostate-specific antigen level
PSA is a single-chain glycoprotein that has chymotrypsinlike properties. PSA slowly hydrolyzes peptide bonds, thereby liquifying semen. The upper limit of normal for PSA is 4 ng/mL. Some advocate age-related cutoffs, such as 2.5 ng/mL for the fifth decade of life, 3.5 ng/mL for the sixth decade of life, and 4.5 ng/mL for the seventh decade of life. Others advocate race-specific reference ranges. Using recent data from screening studies, some have advocated upper limits of normal of 2.5 ng/mL instead of 4 ng/mL.
Prostate-specific antigen velocity
PSA velocity is an important concept. A PSA velocity of lower than 0.75 ng/mL/y has traditionally been used to prompt a prostate biopsy. However, recent data suggest that, among men younger than 50 years, a PSA velocity of 0.6 ng/mL/y may be more appropriate.
Percent of free prostate-specific antigen
The measurement of bound and free PSA is a recent development that can help to differentiate mildly elevated PSA levels due to cancer from elevated levels due to benign prostatic hyperplasia. The lower the ratio of free-to-total PSA, the higher the likelihood of cancer. Free PSA is reported as a percentage. For example, among men with greater than 25% free PSA, only 8% are found to have cancer at prostate biopsy. In contrast, more than half of men with less than 10% free PSA are found to have cancer at biopsy. While cutoffs may be used, the percentage of free PSA is usually used as an additional factor in making an informed recommendation for or against biopsy. Generally, these percentages are useful in patients who have a PSA level in the range of 4-10 ng/mL.
This information is most useful in men with very large glands or in men in whom one biopsy result has already been negative. In healthy men with a PSA level of 4-10 ng/mL, many recommend biopsy without the additional free-PSA test or consider a trial of antibiotic therapy for 4-6 weeks before repeating the PSA test. If antibiotic therapy quickly lowers the PSA level to within the reference range, the cause of the prior elevation is less likely to be prostate cancer, and the PSA test should be repeated within a few months.
Abnormal digital rectal examination findings
Various factors are considered when a DRE is performed. A nodule is important, but findings such as asymmetry, difference in texture, and bogginess are important clues to the patient's condition and should be considered in conjunction with the PSA level. Change in texture over time can offer important clues about the need for intervention. Cysts or stones cannot be accurately differentiated from cancer based on DRE findings alone; therefore, maintain a high index of suspicion if the DRE results are abnormal. In addition, if cancer is detected, the DRE findings form the basis of clinical staging of the primary tumor (ie, tumor [T] stage in the tumor node metastases [TNM] staging system). In current practice, the DRE results are normal but the PSA readings are abnormal in most patients diagnosed with prostate cancer.
Local Symptoms
In the pre-PSA era, patients with prostate cancer commonly presented with local symptoms. Urinary retention developed in 20-25% of these patients, back or leg pain developed in 20-40%, and hematuria developed in 10-15%. Currently, with PSA screening, patients report urinary frequency (38%), decreased urine stream (23%), urinary urgency (10%), and hematuria (1.4%). However, none of these symptoms is unique to prostate cancer and each could arise from various other ailments. Forty-seven percent of patients are asymptomatic.
Metastatic Symptoms
Metastatic symptoms include weight loss and loss of appetite; bone pain, with or without pathologic fracture (because prostate cancer, when metastatic, has a strong predilection for bone); and lower extremity pain and edema due to obstruction of venous and lymphatic tributaries by nodal metastasis. Uremic symptoms can occur from ureteral obstruction caused by local prostate growth or retroperitoneal adenopathy secondary to nodal metastasis.
Frequency
With the advent of PSA screening, a greater number of men require education about prostate cancer and how it is diagnosed, staged, and treated so they can select the most appropriate treatment.
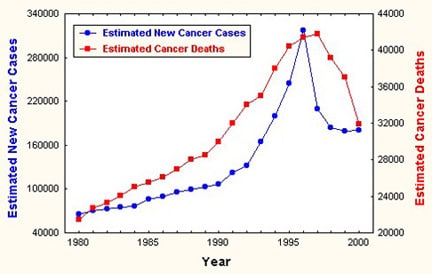
According to figures from the American Cancer Society, 186,330 new cases will be diagnosed in 2008 and 26,000 men will die from prostate cancer (see Image 1). Prostate cancer is rarely diagnosed in men younger than 40 years, and it is uncommon in men younger than 50 years.
Estimated incidence and mortality from prostate cancer. Courtesy of the American Cancer Society.
Prevalence rates of prostate cancer remain significantly higher in African American men than in white men, while the prevalence in Hispanic men is similar to that of white men. Hispanic men and African American men present with more advanced disease, most likely related to external (eg, income, education, insurance status) and cultural factors. In addition, African American men generally have higher levels of testosterone, which may contribute to the higher incidence of carcinoma.
Between 1989 and 1992, incidence rates of prostate cancer increased dramatically, probably because of earlier diagnoses in asymptomatic men as a result of the increased use of serum PSA testing. In fact, the incidence of organ-confined disease at diagnosis has increased because both PSA testing and standard DRE are performed.
Prostate cancer incidence rates are continuing to decline; rates in white men peaked in 1992, and they peaked in African American men in 1993.
During 1992-1996, mortality rates associated with prostate cancer declined significantly, approximately 2.5% per year (see Image 1). Although mortality rates are continuing to decline among white and African American men, mortality rates in African American men remain twice as high as in white men, based on 2008 American Cancer Society projections.
Prostate cancer is also found during autopsies performed following other causes of death. The rate of this latent or autopsy cancer is much greater than that of clinical cancer. In fact, it may be as high as 80% by age 80 years.
The prevalence of clinical cancer varies by region, and these differences may be due to some of the genetic, hormonal, and dietary factors discussed in Etiology. High rates are reported in northern Europe and North America, intermediate rates are reported in southern Europe and Central and South America, and low rates are reported in Eastern Europe and Asia.
Interestingly, the prevalence of the latent or autopsy form of the disease is similar worldwide. Together with migration studies, this suggests that environmental factors, such as diet, may play a significant promoting role in the development of a clinical cancer secondary to a latent precursor.
Etiology
Genetics
Gene alterations on chromosome 1, 17, and the X chromosome have been found in some patients with a family history of prostate cancer. The hereditary prostate cancer 1 (HPC1) gene and the predisposing for cancer of the prostate (PCAP) gene are on chromosome 1, while the human prostate cancer gene is on the X chromosome. In addition, genetic studies suggest that a strong familial predisposition may be responsible for as many as 5-10% of prostate cancer cases. Recently, several reports have suggested a shared familial risk (inherited or environmental) for prostate and breast cancer. Men with a family history of prostate cancer have a higher risk of developing prostate cancer and are also likely to present 6-7 years earlier.
Race
African American men have a higher prevalence and more aggressive prostate cancer than white men, who, in turn, have a higher prevalence than men of Asian origin. Studies have found that young African American men have testosterone levels that are 15% higher than in young white men. Furthermore, evidence indicates that 5-alpha reductase may be more active in African Americans than in whites, implying that hormonal differences may play a role. The independent contribution of race alone is difficult to qualify when the effects of health care access, income, education, and insurance status are also considered.
Diet
A high-fat diet may lead to increased risks, while a diet rich in soy may be protective. These observations have been proposed as reasons for the low prevalence of this cancer in Asia. Rates of prostate cancer are much greater in Japanese American men than in native Japanese men, supporting the association of a high-fat diet with cancer. Cell culture studies have shown that omega-6 fatty acids are positive stimulants of prostate cancer cell growth, while omega-3 fatty acids are negative stimuli. These fats may exert their effects by alterations of sex hormones or growth factors or through effects on 5-alpha reductase.
Soy seems to decrease the growth of prostate cancer cells in mouse models; however, apart from epidemiologic factors, no direct evidence supports a beneficial effect in humans. Vitamin E may have some protective effects because it is an antioxidant. Decreased levels of vitamin A may be a risk factor because this can promote cell differentiation and stimulate the immune system. Vitamin D deficiency was suggested as a risk factor, and studies show an inverse relationship between ultraviolet exposure and mortality rates for prostate cancer. However, a specific correlation between 1,25-dihydroxyvitamin D levels and palpable disease, well-differentiated tumors, or mortality is inconclusive.
Selenium may have a protective effect based on epidemiologic studies and is also believed to extend its effect via its antioxidant properties. The Selenium and Vitamin E Cancer Prevention Trial (SELECT) is an ongoing intergroup, phase 3, randomized, controlled trial designed to test the efficacy of selenium and vitamin E alone and in combination in the prevention of prostate cancer.
For more information, see Prostate Cancer: Nutrition.
Hormones
Hormonal causes have also been postulated. Androgen ablation causes a regression of prostate cancer. In addition, as indirect evidence of hormonal causes, eunuchs do not develop adenocarcinoma of the prostate.
Hsing and Comstock performed a large study comparing patients with prostate cancer with controls and found no difference in levels of testosterone, dehydrotestosterone, prolactin, follicle-stimulating hormone, or estrone.1
The Prostate Cancer Prevention Trial studied the prevalence of prostate cancer between a control group and a group given a 5-alpha-reductase inhibitor (finasteride). While the 5-alpha reductase inhibitor appeared to decrease the prevalence of tumors, those that did arise appeared histologically more aggressive. Only long-term follow-up of these patients will determine whether this more aggressive histology accurately reflects the underlying biology of these tumors or whether it is an artifact of the treatment.
The American Society of Clinical Oncology (ASCO) Health Services Committee (HSC), ASCO Cancer Prevention Committee, and the American Urological Association Practice Guidelines Committee jointly convened a Panel of experts who used the results from a systematic review of the literature to develop evidence-based recommendations on the use of 5-alpha-reductase inhibitors for prostate cancer chemoprevention.The Expert Panel concluded that asymptomatic men with a PSA level of less than 3 ng/mL who are regularly screened with PSA or are anticipating undergoing annual PSA screening for early detection of prostate cancer may benefit from a discussion of both the benefits of 5-alpha-reductase inhibitors for 7 years for the prevention of prostate cancer and the potential risks (including the possibility of high-grade prostate cancer).
Men who are taking 5-alpha-reductase inhibitors for benign conditions, such as lower urinary tract (obstructive) symptoms (LUTS), may benefit from a similar discussion; these patients should understand that the improvement of LUTS relief should be weighed with the potential risks of high-grade prostate cancer from 5-alpha-reductase inhibitors (although most of the Panel members judged the risk of high-grade prostate cancer to be unlikely). A reduction of approximately 50% in PSA level by 12 months is expected in men taking a 5-alpha-reductase inhibitor; however, because these changes in PSA may vary among men, and within individual men over time, the Panel has no recommendations for a specific cut point to trigger a biopsy for men taking a 5-alpha-reductase inhibitor. No specific cut point or change in PSA level has been prospectively validated in men taking a 5-alpha-reductase inhibitor.2
Pathophysiology and Natural History
Pathophysiology
Prostate cancer develops when the rates of cell division and cell death are no longer equal, leading to uncontrolled tumor growth. Following the initial transformation event, further mutations of a multitude of genes, including the genes for p53 and retinoblastoma, can lead to tumor progression and metastasis. Most (95%) prostate cancers are adenocarcinomas.
Approximately 4% of cases of prostate cancer have transitional cell morphology and are thought to arise from the urothelial lining of the prostatic urethra. Few cases have neuroendocrine morphology. When present, they are believed to arise from the neuroendocrine stem cells normally present in the prostate or from aberrant differentiation programs during cell transformation.
Of prostate cancer cases, 70% arise in the peripheral zone, 15-20% arise in the central zone, and 10-15% arise in the transitional zone. Most prostate cancers are multifocal, with synchronous involvement of multiple zones of the prostate, which may be due to clonal and nonclonal tumors.
Natural history
The natural history is still relatively unknown, and many aspects of progression are poorly understood. Symptoms or abnormal DRE findings in the pre-PSA era brought only 40-50% of patients with prostate cancer to medical attention, and these patients usually had locally advanced disease. The advent of PSA testing has helped to identify patients with less-advanced, organ-confined disease.
In fact, the pendulum has shifted to the point that certain members of the urologic community feel that active surveillance, also known as expectant management, may have a role. Twenty-year outcome data from Connecticut confirm that mortality rates due to tumors with a Gleason score of 2-4 was less than 7%.3 Urologists at Johns Hopkins University advocate active surveillance in patients with a PSA density of less than 0.1 ng/mL, with no adverse pathologic findings on needle biopsy, and with tumors with a Gleason score of 6 that are smaller than 3 mm.
Evidence suggests that most prostate cancers are multifocal and heterogeneous. Cancers can start in the transitional zone or, more commonly, the peripheral zone. When these cancers are locally invasive, the transitional-zone tumors spread to the bladder neck, while the peripheral-zone tumors extend into the ejaculatory ducts and seminal vesicles. Penetration through the prostatic capsule and along the perineural or vascular spaces occurs relatively late.
The mechanism for distant metastasis is poorly understood. The cancer spreads to bone early, occasionally without significant lymphadenopathy. Currently, 2 predominant theories have been proposed for spread—the mechanical theory and the seed-and-soil theory.
- The mechanical theory involves direct spread through the lymphatics and venous spaces into the lower lumbar spine.
- Advocates of the seed-and-soil theory believe that tissue factors that allow for preferential growth in certain tissues, such as the bone, must be present. Lung, liver, and adrenal metastases have also been documented. Specific tissue growth factors and extracellular matrices are possible examples.
The doubling time in early-stage disease is as slow as 2-4 years, but this changes as the tumor grows and becomes more aggressive. Larger tumors usually have a higher Gleason grade and a faster doubling time.
Natural history by stage
- T1a - Progression over 10 years (uncommon)
- T1b - Tumor-related death rate of 10% in 10 years
- T2 - Ten-year metastasis-free survival rate of 81% with grade 1, 58% with grade 2, and 26% with grade 3
- T3 - Lymph node metastasis at presentation in 50% and approximately 25% rate of 10-year disease-free survival
The natural history of clinically localized disease varies, with lower-grade tumors having a more indolent course, while some high-grade lesions progress to metastatic disease with relative rapidity. Several studies have examined the cancer-specific and quality-of-life outcomes associated with a watchful-waiting approach to localized disease.
- Albertsen et al monitored patients who received no initial treatment for prostate cancer.3 As disease progression occurred, many received antiandrogens. Men with poorly differentiated tumors lost 6-8 years of life, while those with moderately differentiated tumors lost 4-5 years. Of all men monitored for 10 years, 40% died of causes other than prostate cancer. This study was performed prior to PSA screening.
- Graversen et al compared watchful waiting with radical prostatectomy.4 They found no overall difference in survival, but they did find that a high Gleason score was associated with poor survival in both groups.
- Chodak et al confirmed this finding by analyzing 6 studies and finding a 34% survival rate associated with grade 3 tumors versus an 87% disease-specific survival rate associated with grade 1 and 2 tumors.5 The metastasis-free survival rate also significantly dropped as the grade progressed from 1 to 3.
- Johansson et al (2004) reported their recent update on a population-based cohort study with a mean observation period of 21 years.6 In this study, 223 patients with early-stage, initially untreated prostatic cancer were observed. Symptomatic patients with tumor progression received hormonal treatment (orchiectomy or estrogens). Thirty-nine (17%) developed metastatic disease, with most cancers having an indolent course during the first 10-15 years. However, further follow-up at 15-20 years revealed a substantial decrease in cumulative progression-free survival (from 45% to 36%), survival without metastases (from 76.9% to 51.2%), and prostate cancer–specific survival (from 78.7% to 54.4%). Prostate cancer mortality increased from 15 deaths per 1000 person-years during the first 15 years to 44 deaths per 1000 person-years beyond 15 years of follow-up.
Taken together, these data suggest that, although most prostate cancers diagnosed at an early stage have an indolent course, local tumor progression and aggressive metastatic disease may develop in the long term. In addition, these findings would support early radical treatment, notably among patients with an estimated life expectancy exceeding 15 years.
Screening
DRE and PSA evaluation are the 2 components necessary for a modern screening program. Transrectal ultrasonography (TRUS) has been associated with a high false-positive rate, making it unsuitable as a screening tool, although it is very useful for directing prostatic biopsies.
The indications for screening are controversial. The American Cancer Society recommends that both PSA evaluation and DRE should be offered annually, beginning at age 50 years, to men who have at least a 10-year life expectancy and to high-risk younger men. Information should be provided to patients regarding potential risks and benefits of intervention.
Despite the apparent survival advantage of early diagnosis conferred by PSA screening, a recent U.S. Preventive Services Task Force statement recommends against screening for prostate cancer in men aged 75 years or older. The statement also concludes that, currently, the balance of benefits versus drawbacks of prostate cancer screening in men younger than age 75 years cannot be assessed because of insufficient evidence.7
Advocates of screening believe that early detection is crucial to finding organ-confined disease and to reducing the likelihood of mortality. When symptoms develop or when DRE results become positive, most cases have already advanced beyond organ-confined disease. Those who do not advocate screening worry that screening will detect cancers that are not biologically significant (ie, in patients who will die with prostate cancer rather than from it). Currently, age-specific PSA cutoffs are used to guide screening. The trend is toward lowering the threshold level to 2.5 ng/mL, but this has not yet been widely accepted.
Men who choose to undergo screening should begin at age 50 years. Men in high-risk groups, such as African Americans and those with a strong familial predisposition (2 or more affected first-degree relatives), should begin screening at a younger age (40-45 y). These men are less likely to have the latent form of the disease and benefit from treatment. More data on the precise age to start prostate cancer screening are needed for men at high risk.
Recent data from Canadian and Austrian studies suggest that mortality rates are lower as a result of PSA screening. Canadian data have shown that, from 1989-1996, the mortality rate was lower in the PSA-screened cohort than in the control group. Recent studies from Tyrol, Austria, also show a beneficial result for screening in reducing disease-specific mortality. These beneficial effects are likely due to the fact that treatment rather than observation may enhance disease-specific survival. This was recently shown in a 2002 Scandinavian study, which reported that radical prostatectomy was associated with significantly reduced disease-specific mortality compared with watchful waiting. No difference in overall survival was noted.
Currently, US data have shown a mortality rate decrease of 1% per year since 1990, which coincides with the advent of PSA screening. Other theories have been proposed to account for the decrease, and these include changing treatment practices and artifacts in mortality rates secondary to the changing incidence.
Abnormal rectal examination findings
Findings from the DRE are crucial. An irregular firm prostate or nodule is typical, but many cancers are found in prostates that feel normal. Pay careful attention to the prostate consistency, along with the seminal vesicles and adjacent organs, to detect spread of the disease to these structures.
- Overdistended bladder due to outlet obstruction
- Neurologic findings secondary to cord compression: Other subtle findings, such as paresthesias or wasting, are uncommon.
- Lower extremity lymphedema
- Supraclavicular adenopathy
- Lower extremity deep venous thrombosis
- Cancer cachexia
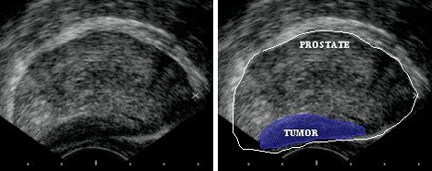
Transrectal ultrasonography
TRUS is used to examine the prostate for hypoechoic areas, which are commonly associated with cancers but are not specific enough for diagnostic purposes. At least 6 or, more recently, 10 or more systematic biopsy specimens of peripheral and, occasionally, transitional zones are taken under ultrasonographic guidance. Samples should include most areas of the gland, irrespective of ultrasonographic abnormalities.
Transrectal sonogram of the prostate showing a hypoechoic lesion in the peripheral zone of the gland that is suggestive of cancer.
Differential diagnoses
- Benign prostatic hypertrophy
- Calculi
- Prostatic cysts
- Prostatic tuberculosis
- Prostatitis
Staging
The 2002 TNM staging system is used to stage prostate cancer, as follows:
- T - Primary tumor
- TX - Primary tumor cannot be assessed
- T0 - No evidence of primary tumor
- T1 - Clinically inapparent tumor not palpable or visible by imaging
- T1a - Tumor incidental histologic finding in less than or equal to 5% of tissue resected
- T1b - Tumor incidental histologic finding in greater than 5% of tissue resected
- T1c - Tumor identified by needle biopsy (because of elevated PSA level); tumors found in 1 or both lobes by needle biopsy but not palpable or reliably visible by imaging
- T2 - Tumor confined within prostate
- T2a - Tumor involving less than half a lobe
- T2b - Tumor involving less than or equal to 1 lobe
- T2c - Tumor involving both lobes
- T3 - Tumor extending through the prostatic capsule; no invasion into the prostatic apex or into, but not beyond, the prostatic capsule
- T3a - Extracapsular extension (unilateral or bilateral)
- T3b - Tumor invading seminal vesicle(s)
- T4 - Tumor fixed or invading adjacent structures other than seminal vesicles (eg, bladder neck, external sphincter, rectum, levator muscles, pelvic wall)
- NX - Regional lymph nodes (cannot be assessed)
- N0 - No regional lymph node metastasis
- N1 - Metastasis in regional lymph node or nodes
Regional lymph nodes are assessed via surgical removal or biopsy of the pelvic lymph nodes, including the obturator chain. The surgical boundaries include the bifurcation of the common iliac, the obturator nerve, and the node of Cloquet.
Distant metastasis
- PM1c - More than 1 site of metastasis present
- MX - Distant metastasis cannot be assessed
- M0 - No distant metastasis
- M1 - Distant metastasis
- M1a - Nonregional lymph node(s)
- M1b - Bone(s)
- M1c - Other site(s)
Workup and Histologic Findings
Workup
Determine the PSA level. Age-related PSA levels can be assessed, as can clinical evidence of prostatitis. If the physician believes that an elevated PSA level may be due to infection, 4-6 weeks of antibiotics are provided, and then the PSA level is rechecked.
Perform a DRE. This is examiner-dependent, and serial examinations, over time, are best. Regard nodules or changes in the texture or the level of asymmetry with a high index of suspicion. Physical examination findings alone cannot reliably differentiate a cyst or calculus from cancer foci; therefore, a biopsy is warranted in these circumstances.
Perform a biopsy to aid in diagnosis and determine the Gleason score. Antibiotics are administered, and an enema is often provided before the procedure, followed by a short course of antibiotics after the biopsy. Coagulation tests are not routinely performed, but patients are instructed to stop aspirin and nonsteroidal anti-inflammatory drugs 10 days prior to the biopsy. Many physicians use lidocaine prior to the biopsy, while others do not. The number of biopsies that should be performed is debated. Sextant versus 12- versus 18-core biopsy protocols are published in the literature. The 12- or 18-core protocols yield more specimens from the lateral regions and usually sample the transition zone. Several studies have demonstrated an increase in the cancer detection rate, while others have not.
In patients with a persistently elevated PSA level in the face of negative biopsy results, the literature supports repeating the biopsy once or twice. Of cancer cases, 31% were detected on repeat biopsy and 39% were detected if the PSA value was greater than 20 ng/mL. If all the biopsy results are negative, a repeat round of biopsies has been suggested when the PSA increases by 25% from the level at which the last biopsies were performed.
Further workup depends on the clinical staging. A higher clinical stage of cancer determined by DRE findings, PSA level, and Gleason score (as determined by biopsy) correlates with an increased risk of extraprostatic spread, and these tests are considered key factors in determining the staging workup and predicting patient prognosis.
The Partin tables are the best nomogram for predicting prostate cancer spread and prognosis. In addition, a series of nomograms has been issued from the Memorial Sloan-Kettering Cancer Center; these nomograms are used to predict biochemical-free survival after surgery and radiation. The most commonly used is the Kattan nomogram.
Men with PSA levels less than 10 ng/mL and low- or moderate-grade histology (Gleason score <7)>>7), or physical findings that suggest stage T3 disease should probably undergo a staging CT scanning and bone scan. CT scanning is the one modality with evidence-based guidelines. The CT scanning can be used to evaluate extension into the bladder and lymph nodes to help stage the patient's cancer or to consider lymph node sampling prior to treatment. TRUS is no better than DRE, and positron emission tomography scans have not been proven effective.
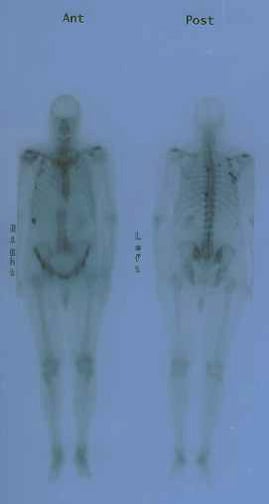
MRI is superior to bone scan in evaluating bone metastasis but is impractical for routine total-body surveys. Instead, it is used to determine the etiology of questionable lesions found on bone scans. MRI is promising for local staging but is not readily accessible, and no published guidelines are available.
Anterior and posterior bone scans of a patient with prostate cancer, with metastasis to the 12th rib and thoracic spine represented by the increased uptake of isotope.
Neither CT scanning nor MRI can be used to determine if lymph nodes are reactive or contain malignant deposits unless the nodes are significantly enlarged and a percutaneous biopsy can be performed.
There is increasing interest in using metabolic activity to detect cancer foci. Positron emission tomography (PET) uses glucose analogue 18 F-fluorodeoxyglucose (18 F-FDG) to detect cancer, but studies thus far have been disappointing for prostate cancer detection.
C-choline PET scans fused with CT imaging show more promise but are not yet the standard of care. Likewise, there is renewed interest in ProstaScint scans fused with MRI or CT images. This modality involves a murine monoclonal antibody that reacts with prostate-specific membrane antigen to identify cancer both in the prostate and in metastatic deposits.
Finally, conventional endorectal MRI is helpful for localizing cancer within the prostate and seminal vesicles and for local staging. Dynamic contrast-enhanced MRI and MR spectroscopic imaging are also complementary in local staging, but their use is currently limited to a research setting.
Preoperative workup includes the following:
- Chest radiography
- CBC count
- CHEM-7
- Prothrombin time and activated partial thromboplastin time
- Electrocardiography
Histologic findings
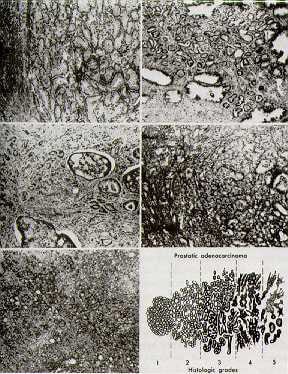
The most commonly used system of classifying histologic characteristics of prostate cancer is the Gleason score, which is determined using the glandular architecture within the tumor.
The predominant pattern and the second most common pattern are given grades from 1-5. The sum of these 2 grades is referred to as the Gleason score. Scoring based on the 2 most common patterns is an attempt to factor in the considerable heterogeneity within cases of prostate cancer. In addition, this scoring method was found to be superior for predicting disease outcomes compared with using the individual grades alone.
Histologic scoring system showing the 2 most common patterns seen on the biopsy specimen, termed the Gleason score.
Grades are based on the extent to which the epithelium assumes a normal glandular structure. A grade of 1 indicates a near-normal pattern, and grade 5 indicates the absence of any glandular pattern (less malignant to more malignant). This scheme of grading histological features greatly depends on the skill and experience of the pathologist and is subject to some degree of individual variation.
- A score of 2-4 is considered low grade or well differentiated.
- A score of 5-7 is considered moderate grade or moderately differentiated.
- A score of 8-10 is considered high grade or poorly differentiated.
Although the change in glandular architecture represented by the Gleason score is currently the most widely used and correlative histological parameter, it is not the only histological change that can be observed in prostate cancers. Indeed, notable changes in cell and nuclear morphology, neuroendocrine differentiation, and vascularity can be observed and may have great prognostic significance.
Perineural invasion is an indicator of invasiveness and is considered in terms of which side should possibly undergo a nerve-sparing procedure and whether a patient might benefit more from high- or low-risk brachytherapy.
Prostatic intraepithelial neoplasia (PIN) represents the putative precancerous end of the morphologic continuum of cellular proliferations within prostatic ducts, ductules, and acini.
Two grades of PIN are identified. Low-grade PIN is mild dysplasia. High-grade PIN encompasses moderate and severe dysplasia. High-grade PIN is considered by most to be a precursor of invasive carcinoma. Men with high-grade PIN alone can be started on finasteride and monitored closely.
The continuum that culminates in high-grade PIN and early invasive cancer is characterized by basal cell layer or basement membrane disruption, progressive loss of secretory differentiation markers, increasing nuclear and nucleolar abnormalities, increasing proliferative potential, and increasing variation in DNA content (aneuploidy).
Clinical studies suggest that PIN predates a carcinoma by 10 or more years. The clinical importance of recognizing PIN is based on its strong association with carcinoma. Recent studies claim that men with high-grade PIN in a prostate biopsy specimen have a 35-50% chance of being diagnosed with prostate cancer after a subsequent biopsy. Atypical small acinar proliferation (ASAP) has also been associated with higher cancer detection rates. The identification of PIN in prostate biopsy specimens warrants further searching for concurrent invasive carcinoma. In most men, this means repeat biopsies if the PSA level changes significantly. The same may also be true for ASAP findings after biopsy.
Relevant Anatomy
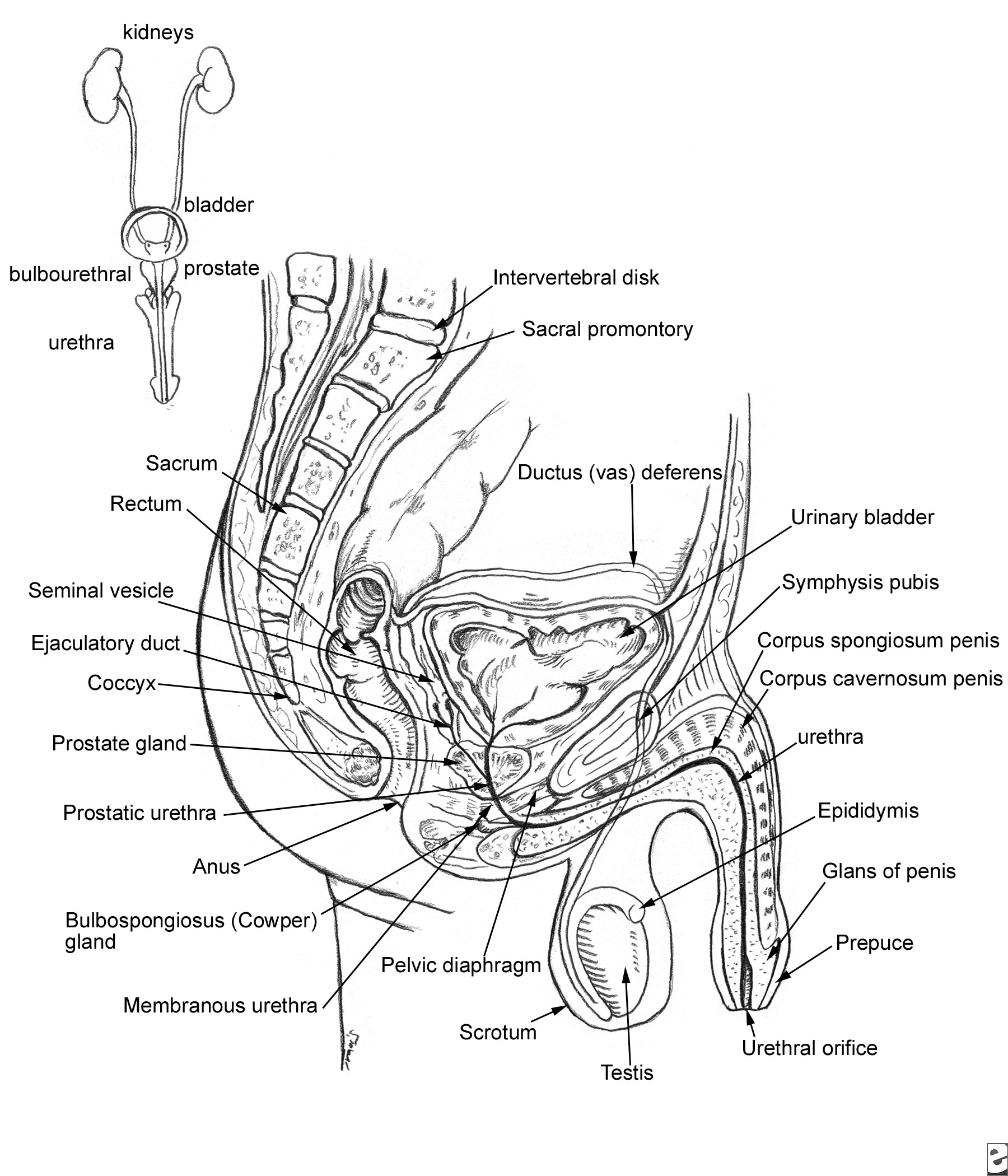
See Image 2. The prostate lies below the bladder and encompasses the prostatic urethra. It is surrounded by a capsule and is separated from the rectum by a layer of fascia termed the Denonvilliers aponeurosis.
Relevant anatomy of the male pelvis and genitourinary tract.
The blood supply to the base of the bladder and prostate is from the inferior vesical, which is derived from the internal iliac. The capsular branches of the inferior vesical artery help identify the pelvic plexus arising from the S2-S4 and T10-T12 nerve roots.
The neurovascular bundle lies on either side of the prostate on the rectum. It is derived from the pelvic plexus and is important for erectile function.
Future and Controversies
Whether one of the several different modalities used for treating localized prostate cancer offers survival benefits over another remains controversial. The choice of definitive therapy has been suggested to make a significant difference in long-term survival in less than 10% of patients. This means that most patients are either cured by any definitive therapy or present with incurable disease that cannot be detected, and, ultimately, any treatment modality fails to be curative.
A 2008 research summary by the Agency for Healthcare Research and Quality (AHRQ) concluded that no single therapy can be considered the preferred treatment for presumed organ-confined prostate cancer. The AHRQ based this conclusion partly on the lack of data regarding efficacy and partly on the concept that differences in adverse effects, convenience, and costs among the available therapies may be important factors in the choice of treatment in an individual patient. The AHRQ noted that, although all treatment options carry adverse effects, patient satisfaction with therapy is high.8
Molecular prognostic markers
Over the past few years, several molecular markers have been shown to aid in the prognostication of patients undergoing treatment for localized and metastatic prostate cancers. Assessment of the molecular alterations or gene products of TP53, RB, BCL2, cathepsin-D, CDH1, and PTEN, among many others, have been reported. Prospective trials are needed to assess these markers more thoroughly before their implementation in current management is recommended.
Reverse transcriptase-polymerase chain reaction
Reverse transcriptase-polymerase chain reaction (RTPCR) testing may be able to find very small amounts of PSA nucleic acid in the blood stream, prostatic fossa, or bone marrow. In the future, this may be helpful in determining which patients have residual tumor following surgery (RTPCR-positive prostate fossa) or a higher rate of tumor recurrence (RTPCR-positive lymph nodes at surgery or persistently positive bone marrow samples months after treatment).
Multimedia
Media file 1: Estimated incidence and mortality from prostate cancer. Courtesy of the American Cancer Society.
Media file 2: Relevant anatomy of the male pelvis and genitourinary tract.
Media file 3: Transrectal sonogram of the prostate showing a hypoechoic lesion in the peripheral zone of the gland that is suggestive of cancer.
Media file 4: Anterior and posterior bone scans of a patient with prostate cancer, with metastasis to the 12th rib and thoracic spine represented by the increased uptake of isotope.
Media file 5: Histologic scoring system showing the 2 most common patterns seen on the biopsy specimen, termed the Gleason score.
References
Hsing AW, Comstock GW. Serological precursors of cancer: serum hormones and risk of subsequent prostate cancer. Cancer Epidemiol Biomarkers Prev. Jan-Feb 1993;2(1):27-32. [Medline].
[Best Evidence] Kramer BS, Hagerty KL, Justman S, Somerfield MR, Albertsen PC, Blot WJ, et al. Use of 5-alpha-reductase inhibitors for prostate cancer chemoprevention: American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. J Clin Oncol. Mar 20 2009;27(9):1502-16. [Medline].
Albertsen PC, Fryback DG, Storer BE, Kolon TF, Fine J. Long-term survival among men with conservatively treated localized prostate cancer. JAMA. Aug 23-30 1995;274(8):626-31. [Medline].
Graversen PH, Nielsen KT, Gasser TC, Corle DK, Madsen PO. Radical prostatectomy versus expectant primary treatment in stages I and II prostatic cancer. A fifteen-year follow-up. Urology. Dec 1990;36(6):493-8. [Medline].
Chodak GW, Thisted RA, Gerber GS, Johansson JE, Adolfsson J, Jones GW, et al. Results of conservative management of clinically localized prostate cancer. N Engl J Med. Jan 27 1994;330(4):242-8. [Medline].
Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, et al. Natural history of early, localized prostate cancer. JAMA. Jun 9 2004;291(22):2713-9. [Medline].
[Best Evidence] U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. Aug 5 2008;149(3):185-91. [Medline].
Agency for Healthcare Research and Quality. Comparative Effectiveness of Therapies for Clinically Localized Prostate Cancer. AHRQ: Agency for Healthcare Research and Quality. Available at http://effectivehealthcare.ahrq.gov/healthInfo.cfm?infotype=rr&ProcessID=9&DocID=79. Accessed January 15, 2009.
Albertsen PC, Fryback DG, Storer BE, Kolon TF, Fine J. The impact of co-morbidity on life expectancy among men with localized prostate cancer. J Urol. Jul 1996;156(1):127-32. [Medline].
Amling CL, Kane CJ, Riffenburgh RH, Ward JF, Roberts JL, Lance RS, et al. Relationship between obesity and race in predicting adverse pathologic variables in patients undergoing radical prostatectomy. Urology. Nov 2001;58(5):723-8. [Medline].
Anastasiadis AG, Sachdev R, Salomon L, Ghafar MA, Stisser BC, Shabsigh R, et al. Comparison of health-related quality of life and prostate-associated symptoms after primary and salvage cryotherapy for prostate cancer. J Cancer Res Clin Oncol. Dec 2003;129(12):676-82. [Medline].
Berthon P, Valeri A, Cohen-Akenine A, Drelon E, Paiss T, Wohr G, et al. Predisposing gene for early-onset prostate cancer, localized on chromosome 1q42.2-43. Am J Hum Genet. Jun 1998;62(6):1416-24. [Medline].
Bostwick DG. Prostatic intraepithelial neoplasia (PIN): current concepts. J Cell Biochem Suppl. 1992;16H:10-9. [Medline].
Bostwick DG, Qian J. High-grade prostatic intraepithelial neoplasia. Mod Pathol. Mar 2004;17(3):360-79. [Medline].
Bratt O, Kristoffersson U, Lundgren R, Olsson H. Familial and hereditary prostate cancer in southern Sweden. A population-based case-control study. Eur J Cancer. Feb 1999;35(2):272-7. [Medline].
Carter BS, Bova GS, Beaty TH, Steinberg GD, Childs B, Isaacs WB, et al. Hereditary prostate cancer: epidemiologic and clinical features. J Urol. Sep 1993;150(3):797-802. [Medline].
Carter HB, Epstein JI, Chan DW, Fozard JL, Pearson JD. Recommended prostate-specific antigen testing intervals for the detection of curable prostate cancer. JAMA. May 14 1997;277(18):1456-60. [Medline].
Carter HB, Pearson JD, Metter EJ, Brant LJ, Chan DW, Andres R, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA. Apr 22-29 1992;267(16):2215-20. [Medline].
Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA. May 14 1997;277(18):1452-5. [Medline].
D'Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. Sep 17 2003;95(18):1376-83. [Medline].
Djavan B, Susani M, Bursa B, Basharkhah A, Simak R, Marberger M. Predictability and significance of multifocal prostate cancer in the radical prostatectomy specimen. Tech Urol. Sep 1999;5(3):139-42. [Medline].
Etzioni R, Legler JM, Feuer EJ, Merrill RM, Cronin KA, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer--part III: Quantifying the link between population prostate-specific antigen testing and recent declines in prostate cancer mortality. J Natl Cancer Inst. Jun 16 1999;91(12):1033-9. [Medline].
Feuer EJ, Merrill RM, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer--part II: Cause of death misclassification and the recent rise and fall in prostate cancer mortality. J Natl Cancer Inst. Jun 16 1999;91(12):1025-32. [Medline].
Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. Jul 27 2005;294(4):433-9. [Medline].
Fuchsjäger M, Shukla-Dave A, Akin O, Barentsz J, Hricak H. Prostate cancer imaging. Acta Radiol. Feb 2008;49(1):107-20. [Medline].
Fuchsjäger M, Shukla-Dave A, Akin O, Barentsz J, Hricak H. The role of imaging in the oncological prostate. Acta Radiol. Feb/2008;49(1):107-20. [Medline].
Gion M, Mione R, Barioli P, Barichello M, Zattoni F, Prayer-Galetti T. Percent free prostate-specific antigen in assessing the probability of prostate cancer under optimal analytical conditions. Clin Chem. Dec 1998;44(12):2462-70. [Medline].
Gleason DF. Histologic grading of prostate cancer: a perspective. Hum Pathol. Mar 1992;23(3):273-9. [Medline].
Greene FL, Sobin LH. The TNM system: our language for cancer care. J Surg Oncol. Jul 2002;80(3):119-20. [Medline].
Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. Aug 2001;28(3):555-65. [Medline].
Hoffman RM, Gilliland FD, Eley JW, Harlan LC, Stephenson RA, Stanford JL, et al. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. Mar 7 2001;93(5):388-95. [Medline].
Holmberg L, Bill-Axelson A, Helgesen F, Salo JO, Folmerz P, Hggman M, et al. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med. Sep 12 2002;347(11):781-9. [Medline].
Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. Jan 1 2000;85(1):60-7. [Medline].
Iczkowski KA, Bassler TJ, Schwob VS, Bassler IC, Kunnel BS, Orozco RE, et al. Diagnosis of "suspicious for malignancy" in prostate biopsies: predictive value for cancer. Urology. May 1998;51(5):749-57; discussion 757-8. [Medline].
Iczkowski KA, Chen HM, Yang XJ, Beach RA. Prostate cancer diagnosed after initial biopsy with atypical small acinar proliferation suspicious for malignancy is similar to cancer found on initial biopsy. Urology. Nov 2002;60(5):851-4. [Medline].
Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. Jan-Feb 2003;53(1):5-26. [Medline].
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. Jan-Feb 2007;57(1):43-66. [Medline].
Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, et al. SELECT: the Selenium and Vitamin E Cancer Prevention Trial: rationale and design. Prostate Cancer Prostatic Dis. Nov 2000;3(3):145-151. [Medline].
Kolonel LN, Nomura AM, Cooney RV. Dietary fat and prostate cancer: current status. J Natl Cancer Inst. Mar 3 1999;91(5):414-28. [Medline].
Labrie F, Candas B, Dupont A, Cusan L, Gomez JL, Suburu RE, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. Feb 1 1999;38(2):83-91. [Medline].
Langer JE, Rovner ES, Coleman BG, et al. Strategy for repeat biopsy of patients with prostatic intraepithelial neoplasia detected by prostate needle biopsy. J Urol. Jan 1996;155(1):228-31. [Medline].
Lee F, Siders DB, Torp-Pedersen ST, Kirscht JL, McHugh TA, Mitchell AE. Prostate cancer: transrectal ultrasound and pathology comparison. A preliminary study of outer gland (peripheral and central zones) and inner gland (transition zone) cancer. Cancer. Feb 15 1991;67(4 Suppl):1132-42. [Medline].
Loeb S, Catalona WJ. Prostate-specific antigen in clinical practice. Cancer Lett. Apr 28 2007;249(1):30-9. [Medline].
McCahy PJ, Harris CA, Neal DE. Breast and prostate cancer in the relatives of men with prostate cancer. Br J Urol. Oct 1996;78(4):552-6. [Medline].
Morgan TO, Jacobsen SJ, McCarthy WF, Jacobson DJ, McLeod DG, Moul JW. Age-specific reference ranges for prostate-specific antigen in black men. N Engl J Med. Aug 1 1996;335(5):304-10. [Medline].
Moyad MA. Soy, disease prevention, and prostate cancer. Semin Urol Oncol. May 1999;17(2):97-102. [Medline].
Ndubuisi SC, Kofie VY, Andoh JY, Schwartz FM. Black-white differences in the stage at presentation of prostate cancer in the District of Columbia. Urology. Jul 1995;46(1):71-7. [Medline].
Prostate Cancer Trialists' Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists' Collaborative Group. Lancet. Apr 29 2000;355(9214):1491-8. [Medline].
Punglia RS, D'Amico AV, Catalona WJ, Roehl KA, Kuntz KM. Effect of verification bias on screening for prostate cancer by measurement of prostate-specific antigen. N Engl J Med. Jul 24 2003;349(4):335-42. [Medline].
Rodriguez C, Calle EE, Tatham LM, Wingo PA, Miracle-McMahill HL, Thun MJ, et al. Family history of breast cancer as a predictor for fatal prostate cancer. Epidemiology. Sep 1998;9(5):525-9. [Medline].
Ruijter ET, Miller GJ, van de Kaa CA, van Bokhoven A, Bussemakers MJ, Debruyne FM, et al. Molecular analysis of multifocal prostate cancer lesions. J Pathol. Jul 1999;188(3):271-7. [Medline].
Sellers TA, Potter JD, Rich SS, Drinkard CR, Bostick RM, Kushi LH, et al. Familial clustering of breast and prostate cancers and risk of postmenopausal breast cancer. J Natl Cancer Inst. Dec 21 1994;86(24):1860-5. [Medline].
Slovin SF, Wilton AS, Heller G, Scher HI. Time to detectable metastatic disease in patients with rising prostate-specific antigen values following surgery or radiation therapy. Clin Cancer Res. Dec 15 2005;11(24 Pt 1):8669-73. [Medline].
Smith JR, Freije D, Carpten JD, Grönberg H, Xu J, Isaacs SD, et al. Major susceptibility locus for prostate cancer on chromosome 1 suggested by a genome-wide search. Science. Nov 22 1996;274(5291):1371-4. [Medline].
Stemmermann GN, Nomura AM, Chyou PH, Yatani R. A prospective comparison of prostate cancer at autopsy and as a clinical event: the Hawaii Japanese experience. Cancer Epidemiol Biomarkers Prev. Mar-Apr 1992;1(3):189-93. [Medline].
Stephenson AJ, Shariat SF, Zelefsky MJ, Kattan MW, Butler EB, Teh BS, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. Mar 17 2004;291(11):1325-32. [Medline].
Theodorescu D, Broder SR, Boyd JC, Mills SE, Frierson HF Jr. p53, bcl-2 and retinoblastoma proteins as long-term prognostic markers in localized carcinoma of the prostate. J Urol. Jul 1997;158(1):131-7. [Medline].
Theodorescu D, Frierson HF Jr, Sikes RA. Molecular determination of surgical margins using fossa biopsies at radical prostatectomy. J Urol. May 1999;161(5):1442-8. [Medline].
Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. Jul 17 2003;349(3):215-24. [Medline].
Walsh PC, Vaughan ED, Retik AB, Wein A, eds. Campbell's Urology. Vol 3. 7th ed. Philadelphia, Pa: WB Saunders; 1998:2487-656.
Warlick CA, Allaf ME, Carter HB. Expectant treatment with curative intent in the prostate-specific antigen era: triggers for definitive therapy. Urol Oncol. Jan-Feb 2006;24(1):51-7. [Medline].
Weinrich MC, Jacobsen SJ, Weinrich SP, Moul JW, Oesterling JE, Jacobson D, et al. Reference ranges for serum prostate-specific antigen in black and white men without cancer. Urology. Dec 1998;52(6):967-73. [Medline].
Whitmore WF Jr. Expectant management of clinically localized prostatic cancer. Semin Oncol. Oct 1994;21(5):560-8. [Medline].
Xu J, Meyers D, Freije D, Isaacs S, Wiley K, Nusskern D, et al. Evidence for a prostate cancer susceptibility locus on the X chromosome. Nat Genet. Oct 1998;20(2):175-9. [Medline].
Yatani R, Shiraishi T, Nakakuki K, et al. Trends in frequency of latent prostate carcinoma in Japan from 1965-1979 to 1982-1986. J Natl Cancer Inst. Jul 6 1988;80(9):683-7. [Medline].
Zhou P, Chen MH, McLeod D, Carroll PR, Moul JW, D'Amico AV. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. Oct 1 2005;23(28):6992-8. [Medline].
Zimmerman SM. Factors influencing Hispanic participation in prostate cancer screening. Oncol Nurs Forum. Apr 1997;24(3):499-504. [Medline].
Keywords
prostate adenocarcinoma, adenocarcinoma of the prostate, prostatic cancer, prostate symptoms, prostate cancer screening, prostate-specific antigen, PSA, benign prostatic hypertrophy, BPH, calculi, prostatic cysts, prostatic tuberculosis, prostatitis, prostate cancer biology, prostate cancer natural history, prostate cancer etiology, prostate cancer staging, TNM staging, Gleason score, PSA score
Contributor Information and Disclosures
Author
Dan Theodorescu, MD, PhD, Paul Mellon Professor of Urologic Oncology, Department of Urology, University of Virginia Health Sciences Center
Dan Theodorescu, MD, PhD is a member of the following medical societies: American Cancer Society, American College of Surgeons, American Urological Association, Medical Society of Virginia, Society for Basic Urologic Research, and Society of Urologic Oncology
Disclosure: Nothing to disclose.
Coauthor(s)
Tracey L Krupski, MD, MPH, Assistant Professor, Department of Urology, University of Virginia
Tracey L Krupski, MD, MPH is a member of the following medical societies: American Medical Association, American Society of Clinical Oncology, American Urological Association, and Society of Women in Urology
Disclosure: Nothing to disclose.
Medical Editor
Edward David Kim, MD, FACS, Professor of Surgery, Division of Urology, University of Tennessee Graduate School of Medicine; Consulting Staff, University of Tennessee Medical Center
Edward David Kim, MD, FACS is a member of the following medical societies: American College of Surgeons, American Society for Reproductive Medicine, American Society of Andrology, American Urological Association, and Tennessee Medical Association
Disclosure: Lilly Consulting fee Consulting; Astellas Consulting fee Speaking and teaching; Indevus Consulting fee Speaking and teaching
Pharmacy Editor
Francisco Talavera, PharmD, PhD, Senior Pharmacy Editor, eMedicine
Disclosure: Nothing to disclose.
Managing Editor
Martin I Resnick, MD †, Former Lester Persky Professor and Chair, Department of Urology, Former Professor, Department of Oncology, Case Western Reserve University School of Medicine
Martin I Resnick, MD † is a member of the following medical societies: American College of Surgeons, American Federation for Medical Research, American Institute of Ultrasound in Medicine, American Medical Association, American Society for Bone and Mineral Research, American Society for Reproductive Medicine, American Society of Andrology, American Surgical Association, American Urological Association, Association for Academic Surgery, Endocrine Society, National Kidney Foundation, Ohio Urological Society, and Pan American Medical Association
Disclosure: Nothing to disclose.
CME Editor
J Stuart Wolf Jr, MD, FACS, David A Bloom Professor of Urology, Director of Division of Minimally Invasive Urology, Department of Urology, University of Michigan
J Stuart Wolf Jr, MD, FACS is a member of the following medical societies: American College of Surgeons, American Urological Association, Catholic Medical Association, Endourological Society, Society for Urology and Engineering, Society of Laparoendoscopic Surgeons, Society of University Urologists, and Society of Urologic Oncology
Disclosure: Terumo Corporation Consulting fee Consulting; Omeros Corporation Consulting fee Consulting
Chief Editor
Edward David Kim, MD, FACS, Professor of Surgery, Division of Urology, University of Tennessee Graduate School of Medicine; Consulting Staff, University of Tennessee Medical Center
Edward David Kim, MD, FACS is a member of the following medical societies: American College of Surgeons, American Society for Reproductive Medicine, American Society of Andrology, American Urological Association, and Tennessee Medical Association
Disclosure: Lilly Consulting fee Consulting; Astellas Consulting fee Speaking and teaching; Indevus Consulting fee Speaking and teaching
 Proponer una traducción mejor
Proponer una traducción mejor